In contact with wound exudates, the hydrocolloid particles gel and interact with the petrolatum component of UrgoTul to form a lipido-colloid gel in contact with the wound, creating a moist environment that promotes the healing process. UrgoTul has pro-healing efficacy, stimulating the proliferation of fibroblasts, key cells in the wound healing process(1,2,3).
Greasy in terms of its chemical composition without being greasy to the touch, UrgoTul does not stick to the wound or its edges: dressing changes are painless and atraumatic for the wound.
This results in some specific properties:
- maintenance of a high level of greasy coating over time(4)
- removal that causes no damage to newly formed tissue(5)
- painless dressing changes for the patient
In contact with wound exudates, the hydrocolloid particles gel and interact with the petrolatum component of UrgoTul to form a lipido-colloid gel in contact with the wound, creating a moist environment that promotes the healing process. UrgoTul has pro-healing efficacy, stimulating the proliferation of fibroblasts, key cells in the wound healing process(1,2,3).
Greasy in terms of its chemical composition without being greasy to the touch, UrgoTul does not stick to the wound or its edges: dressing changes are painless and atraumatic for the wound.
This results in some specific properties:
- maintenance of a high level of greasy coating over time(4)
- removal that causes no damage to newly formed tissue(5)
- painless dressing changes for the patient
In contact with wound exudates, the hydrocolloid particles gel and interact with the petrolatum component of UrgoTul to form a lipido-colloid gel in contact with the wound, creating a moist environment that promotes the healing process. UrgoTul has pro-healing efficacy, stimulating the proliferation of fibroblasts, key cells in the wound healing process(1,2,3).
Greasy in terms of its chemical composition without being greasy to the touch, UrgoTul does not stick to the wound or its edges: dressing changes are painless and atraumatic for the wound.
This results in some specific properties:
- maintenance of a high level of greasy coating over time(4)
- removal that causes no damage to newly formed tissue(5)
- painless dressing changes for the patient
UrgoTul is indicated in the treatment of:
- Acute wounds (burns, dermal abrasions, traumatic wounds, postoperative wounds), chronic wounds (pressure ulcers, diabetic foot ulcers) at the granulation and epithelialisation stage.
- Congenital epidermolysis bullosa wounds.
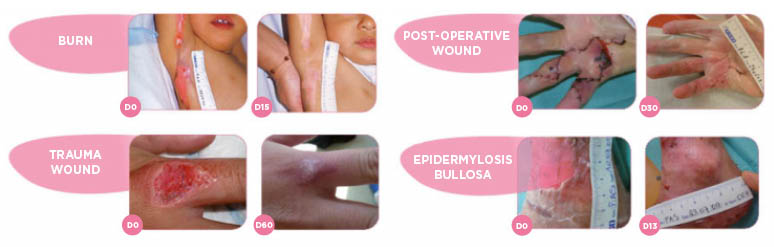
* Traumatic wound: wound treated with UrgoTul Border.
Flexible and highly conformable, UrgoTul is particularly indicated to cover irregular wounds or wounds in difficult locations, as well as for packing deep wounds.
Contraindications:
- Known sensitivity to the dressing.
UrgoTul is indicated in the treatment of:
- Acute wounds (burns, dermal abrasions, traumatic wounds, postoperative wounds), chronic wounds (pressure ulcers, diabetic foot ulcers) at the granulation and epithelialisation stage.
- Congenital epidermolysis bullosa wounds.

* Traumatic wound: wound treated with UrgoTul Border.
Flexible and highly conformable, UrgoTul is particularly indicated to cover irregular wounds or wounds in difficult locations, as well as for packing deep wounds.
Contraindications:
- Known sensitivity to the dressing.
UrgoTul is indicated in the treatment of:
- Acute wounds (burns, dermal abrasions, traumatic wounds, postoperative wounds), chronic wounds (pressure ulcers, diabetic foot ulcers) at the granulation and epithelialisation stage.
- Congenital epidermolysis bullosa wounds.

* Traumatic wound: wound treated with UrgoTul Border.
Flexible and highly conformable, UrgoTul is particularly indicated to cover irregular wounds or wounds in difficult locations, as well as for packing deep wounds.
Contraindications:
- Known sensitivity to the dressing.
- Clean the wound using the conventional care protocol.
- If an antiseptic has previously been used, rinse the wound carefully with normal saline before applying UrgoTul.
- Remove the protective tabs of UrgoTul.
- Apply UrgoTul directly to the wound.
- Cover UrgoTul with a secondary dressing (sterile pads).
- Secure the secondary dressing using a stretchy bandage, multi-stretch adhesive tape or a tubular mesh.
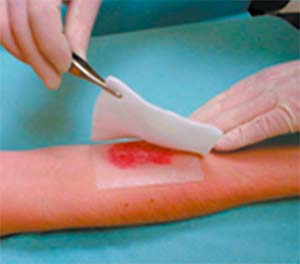
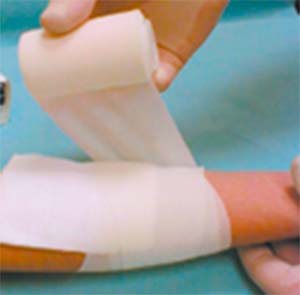
- UrgoTul sticks to latex surgical gloves. It is therefore recommended that gloves be moistened with normal saline to facilitate handling of UrgoTul in this case.
- In the event of clinical signs of local infection, the doctor may decide to continue treatment using an antibacterial dressing.
- In the event of deep, irregular or fistular wounds, leave part of the dressing visible and accessible outside the wound.
- UrgoTul must not be used during hyperbaric oxygen chamber therapy without an oxygen mask (risk of combustion due to the presence of fat). This contraindication does not apply for hyperbaric oxygen chamber therapy with an oxygen mask if the oxygen concentration inside the chamber is less than 25% and if UrgoTul is not applied on the area over which the mask is placed.
- Sterile individual packaging, for single use: reuse of a disposable dressing can lead to risks of infection.
- Check that the sterility protector is intact before use. Do not use if the packaging is damaged.
- Do not re-sterilise the dressing.
- Clean the wound using the conventional care protocol.
- If an antiseptic has previously been used, rinse the wound carefully with normal saline before applying UrgoTul.
- Remove the protective tabs of UrgoTul.
- Apply UrgoTul directly to the wound.
- Cover UrgoTul with a secondary dressing (sterile pads).
- Secure the secondary dressing using a stretchy bandage, multi-stretch adhesive tape or a tubular mesh.


- UrgoTul sticks to latex surgical gloves. It is therefore recommended that gloves be moistened with normal saline to facilitate handling of UrgoTul in this case.
- In the event of clinical signs of local infection, the doctor may decide to continue treatment using an antibacterial dressing.
- In the event of deep, irregular or fistular wounds, leave part of the dressing visible and accessible outside the wound.
- UrgoTul must not be used during hyperbaric oxygen chamber therapy without an oxygen mask (risk of combustion due to the presence of fat). This contraindication does not apply for hyperbaric oxygen chamber therapy with an oxygen mask if the oxygen concentration inside the chamber is less than 25% and if UrgoTul is not applied on the area over which the mask is placed.
- Sterile individual packaging, for single use: reuse of a disposable dressing can lead to risks of infection.
- Check that the sterility protector is intact before use. Do not use if the packaging is damaged.
- Do not re-sterilise the dressing.
- Clean the wound using the conventional care protocol.
- If an antiseptic has previously been used, rinse the wound carefully with normal saline before applying UrgoTul.
- Remove the protective tabs of UrgoTul.
- Apply UrgoTul directly to the wound.
- Cover UrgoTul with a secondary dressing (sterile pads).
- Secure the secondary dressing using a stretchy bandage, multi-stretch adhesive tape or a tubular mesh.


- UrgoTul sticks to latex surgical gloves. It is therefore recommended that gloves be moistened with normal saline to facilitate handling of UrgoTul in this case.
- In the event of clinical signs of local infection, the doctor may decide to continue treatment using an antibacterial dressing.
- In the event of deep, irregular or fistular wounds, leave part of the dressing visible and accessible outside the wound.
- UrgoTul must not be used during hyperbaric oxygen chamber therapy without an oxygen mask (risk of combustion due to the presence of fat). This contraindication does not apply for hyperbaric oxygen chamber therapy with an oxygen mask if the oxygen concentration inside the chamber is less than 25% and if UrgoTul is not applied on the area over which the mask is placed.
- Sterile individual packaging, for single use: reuse of a disposable dressing can lead to risks of infection.
- Check that the sterility protector is intact before use. Do not use if the packaging is damaged.
- Do not re-sterilise the dressing.
(1) Meaume S. Urgotul: a novel non-adherent lipidocolloid dressing. British Journal of Nursing. 2002, Vol 11, issue 16.
(2) Bernard FX et al. Effets d’un pansement lipidocolloïde sur la production de matrice extracellulaire. Journal des Plaies et Cicatrisations, 2007 (study conducted on Urgotul).
(3) Bernard FX et al. Stimulation of the proliferation of human dermal fibroblasts in vitro by a lipidocolloïd dressing. Journal of Wound Care, May 2005; 14 (5): 215-220 (study conducted on Urgotul).
(4) Le Berre. Y. Lurton et al., Pansments imprégnés : tulles/interfaces. CPC 2005 poster, Paris
(5) Meaume S et al. The importance of pain reduction through dressing selection in routine wound manager the MAPP study. Journal of Care 2004, vol 13, No 10, 409-413 (study conducted on UrgoTul).
References:
*Excluding dry necrosis.
**TLC-NOSF: Lipido-Colloid technology – Nano OligaSaccharide Factor (KSOS: Potassium octasulfate sucrose salt).
1. National Institute for Health and Care Excellence (NICE), UrgoStart for treating leg ulcers and diabetic foot ulcers, https://www.nice.org.uk/guidance/mtg42, April 2023
2. Report to the French minister responsible for Social Security and the French Parliament concerning the evolution of French National Health Insurance charges and products for 2014. July 2013. CNAM database: leg ulcers: 210 days; pressure ulcers: 223 days; diabetic foot ulcers: comparative data not available.Edmonds M et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar; 6(3): 186-196.
3. Lázaro-Martínez JL et al. Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer. J Wound Care. 2019 Jun 2; 28(6): 358-367.
4. Münter KC et al. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb; 26 (Sup2): S4-S15. Erratum in: J Wound Care. 2017 Mar 2; 26(3): 153.
5. Report to the French minister responsible for Social Security and the French Parliament concerning the evolution of French National Health Insurance charges and products for 2014. July 2013. CNAM database: leg ulcers: 210 days; pressure ulcers: 223 days; diabetic foot ulcers: comparative data not available.
6. Meaume S et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012; 20(4): 500-511.
7. Dissemond J. et al. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1,140 patients. J Wound Care. 2020; 29(6): 350-361.
8. CAV III: in non-infected (IDSA/IWGDF infection criteria) neuro-ischaemic diabetic foot ulcers (non-critical ischaemia), in the granulation phase (sequential treatment).
9. In the treatment of diabetic foot ulcers. IWGDF Guidelines on the prevention and management of diabetic foot disease, 2019UrgoStart Plus Pad: Class IIb medical device (G-Med; 0459). Treatment to reduce healing time. LPPR fully reimbursable (Social Security: 60% + top-up mutual insurance: 40%) in the treatment of venous or mixed, predominantly venous leg ulcers in the granulation phase (sequential treatment), and in non-infected (IDSA/IWGDF infection criteria) neuro-ischaemic diabetic foot ulcers (non-critical ischaemia), in the granulation phase (sequential treatment). Read the leaflet carefully before use, particularly the precautions for use and contraindications.Manufacturer: Laboratoires URGO – 12/2020.
1. National Institute for Health and Care Excellence (NICE), UrgoStart for treating leg ulcers and diabetic foot ulcers, https://www.nice.org.uk/guidance/mtg42, April 2023
2. “In vitro” study. Internal Report. Laboratoires URGO.
3. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196.
4. Sigal ML, Addala A, Maillard H, Chahim M, Sala F, Blaise S, Dalac S, Meaume S, Bohbot S, Tumba C, Tacca O. Clinical evaluation of a new TLC-NOSF dressing with poly-absorbent fibers for the local management of exuding leg ulcers, at the different stages of the healing process: Results from two multicentric, single-arm, prospective, open-label clinical trials. J Wound Care 2019: 28(3) :164-175
5. Meaume S, Dissemond J, Addala A. Evaluation of two fibrous wound dressings for the management of leg ulcers: results of a European randomised controlled trial (EARTH RCT). J Wound Care 2014; 23: 3, 105–116.
6. Meaume S, Truchetet F, Cambazard F et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012; 20: 4, 500–511.
7. Münter KC, Meaume S, Augustin M, Senet P, Kérihuel J.C. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb; 26 (Sup2): S4-S15. Erratum in: J Wound Care. 2017 Mar 2; 26(3): 153
8. Dissemond J, Lützkendorf S, Dietlein M, Neßeler I, Becker E, Möller U, Thomassin L, Bohbot S, Münter KC. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care. 2020 Jun 2;29(6):350-361. doi: 10.12968/jowc.2020.29.6.350. PMID: 32530781.
9. Augustin M, Keuthage W, Lobmann R, Lützkendorf S, Groth H, Möller U, Thomassin L, Bohbot S, Dissemond J, Blome C. Clinical evaluation of UrgoStart Plus dressings in real-life conditions: results of a prospective multicentre study on 961 patients. J Wound Care. 2021 Dec 2;30(12):966-978. doi: 10.12968/jowc.2021.30.12.966. PMID: 34881999.
10. Lazaro et al . Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer.JWC VOL 28, NO 6 , June 2019
11. Report to the French minister responsible for Social Security and the French Parliament concerning the evolution of French National Health Insurance charges and products for 2014. July 2013.
12. CAV lll: in non-infected (IDSA/IWGDF infection criteria) neuro-ischaemic diabetic foot ulcers (non-critical ischaemia, in the granulation phase (sequential treatment)
13. IWGDF – Guidelines on the prevention and management of diabetic foot disease – 2019.
1. Report to the French minister responsible for Social Security and the French Parliament concerning the evolution of French National Health Insurance charges and products for 2014. July 2013.
2. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196.
3. Meaume S, Truchetet F, Cambazard F et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012; 20: 4, 500–511.
4. Meaume S, Dompmartin A, Lazareth I, Sigal M, Truchetet F, Sauvadet A, Bohbot S. Quality of life in patients with leg ulcers: results from CHALLENGE, a double-blind randomized controlled trial. Journal of Wound Care. 2017; 26 (7): 368-379.
5. Münter KC, Meaume S, Augustin M, Senet P, Kérihuel J.C. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb; 26 (Sup2): S4-S15. Erratum in: J Wound Care. 2017 Mar 2; 26(3): 153.

