The UrgoTul Family range of dressings are for patients whose skin needs that extra TLC. The Technology Lipido-Colloid (TLC) healing matrix:
- Provides and maintains a moist environment to support wound healing
- Ensures harm-free care for patients


Give every patients that extra bit of TLC to support with wound healing
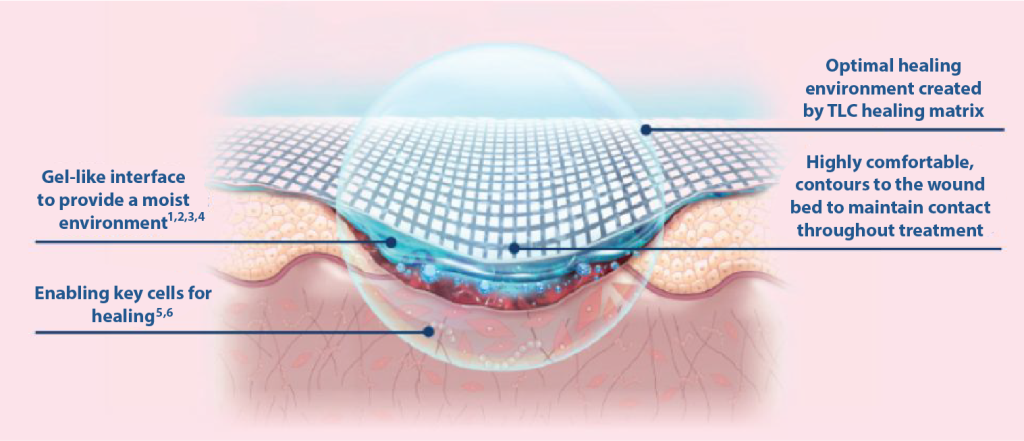
Mode of action where the TLC (Technology Lipido-Colloid) Healing Matrix is in contact with the wound
Specifically designed to improve clinical outcomes


- Maintains a moist environment
- Allows pain-free, atraumatic removal



Absorbent polyurethane foam
- Protects the peri-wound skin against maceration
TLC Healing Matrix
- Maintains a moist environment
- Allows pain-free, atraumatic removal

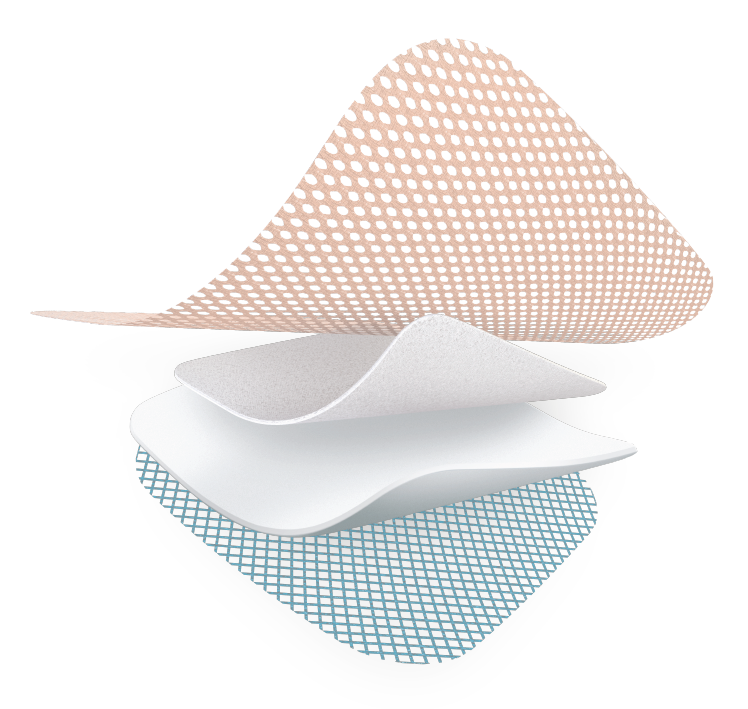


Silicone backing
- Easy to apply & Ready-to-use
- Stays in place for up to 7 days
- Shower-proof
Highly* absorbant layer
- Absorbs and retains exudate
Absorbent polyurethane foam
- Protects the peri-wound skin against maceration
TLC Healing Matrix
- Maintains a moist environment
- Allows pain-free, atraumatic removal
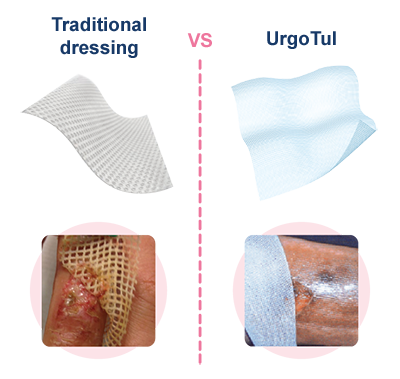
MAPP study (2004).9

We are the only wound care company accredited by the British Skin Foundation
Recognised after a robust, independent review of our extensive research and clinical data
The accreditation demonstrates our commitment to creating products that are not detrimental to a patient’s skin health and our dedication to being the healing company.
1. UrgoStart for treating leg ulcers and diabetic foot ulcers, https://www.nice.org.uk/guidance/mtg42, January 2019
2. IWGDF- Guidelines on the prevention and management of diabetic foot disease – 2019.
3. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196.
4. Meaume S, Truchetet F, Cambazard F et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012; 20: 4, 500–511.
5. Meaume S, Dompmartin A, Lazareth I, Sigal M, Truchetet F, Sauvadet A, Bohbot S. Quality of life in patients with leg ulcers: results from CHALLENGE, a double-blind randomized controlled trial. Journal of Wound Care. 2017; 26 (7): 368-379.
6. Schmutz J.L. et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers : results of a randomised, controlled trial. Int Wound J 2008;5:172–182
7. Sigal ML, Addala A, Maillard H, Chahim M, Sala F, Blaise S, Dalac S, Meaume S, Bohbot S, Tumba C, Tacca O. Clinical evaluation of a new TLC-NOSF dressing with poly-absorbent fibers for the local management of exuding leg ulcers, at the different stages of the healing process: Results from two multicentric, single-arm, prospective, open-label clinical trials. J Wound Care 2019: 28(3) :164-175.
8. Münter KC, Meaume S, Augustin M, Senet P, Kérihuel J.C. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb; 26 (Sup2): S4-S15. Erratum in: J Wound Care. 2017 Mar 2; 26(3): 153
9. Dissemond J, Lützkendorf S, Dietlein M, Neßeler I, Becker E, Möller U, Thomassin L, Bohbot S, Münter KC. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care. 2020 Jun 2;29(6):350-361. doi: 10.12968/jowc.2020.29.6.350. PMID: 32530781.
10. Augustin M, Keuthage W, Lobmann R, Lützkendorf S, Groth H, Möller U, Thomassin L, Bohbot S, Dissemond J, Blome C. Clinical evaluation of UrgoStart Plus dressings in real-life conditions: results of a prospective multicentre study on 961 patients. J Wound Care. 2021 Dec 2;30(12):966-978. doi: 10.12968/jowc.2021.30.12.966. PMID: 34881999.
11. “In vitro” study. Internal Report. Laboratoires URGO.
