
- Fast action: from 30 minutes on main strains
- Works against bacterial strains resistant to antibiotics (e.g MRSA, VRE)
- 99.99% efficacy in just 24h
- Restores the healing process

Complete & continuous cleaning action

![]()
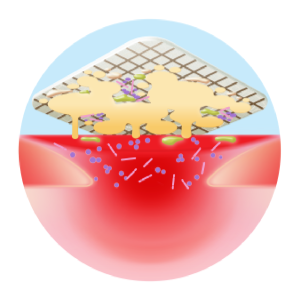
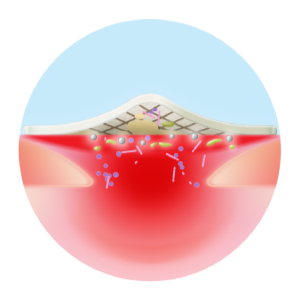
Antibiofilm activity
Antimicrobial activity


Soft adherent pad



Polyabsorbent Fibres
- Delivers a complete and continuous cleaning action
- Absorbs exudate
- Traps slough and maintains a clean wound, optimising silver efficacy
- Disrupts biofilms and prevents their reattachment

TLC-Ag Technology
- Proven antimicrobial efficacy
- Maintains a moist environment
- Allows pain-free, atraumatic removal

Contact layer

Recommended for wounds with <30% sloughy tissue.

TLC-Ag Technology
- Proven antimicrobial efficacy
- Maintains a moist environment
- Allows pain-free, atraumatic removal
1. UrgoStart for treating leg ulcers and diabetic foot ulcers, https://www.nice.org.uk/guidance/mtg42, January 2019
2. IWGDF- Guidelines on the prevention and management of diabetic foot disease – 2019.
3. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196.
4. Meaume S, Truchetet F, Cambazard F et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012; 20: 4, 500–511.
5. Meaume S, Dompmartin A, Lazareth I, Sigal M, Truchetet F, Sauvadet A, Bohbot S. Quality of life in patients with leg ulcers: results from CHALLENGE, a double-blind randomized controlled trial. Journal of Wound Care. 2017; 26 (7): 368-379.
6. Schmutz J.L. et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers : results of a randomised, controlled trial. Int Wound J 2008;5:172–182
7. Sigal ML, Addala A, Maillard H, Chahim M, Sala F, Blaise S, Dalac S, Meaume S, Bohbot S, Tumba C, Tacca O. Clinical evaluation of a new TLC-NOSF dressing with poly-absorbent fibers for the local management of exuding leg ulcers, at the different stages of the healing process: Results from two multicentric, single-arm, prospective, open-label clinical trials. J Wound Care 2019: 28(3) :164-175.
8. Münter KC, Meaume S, Augustin M, Senet P, Kérihuel J.C. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb; 26 (Sup2): S4-S15. Erratum in: J Wound Care. 2017 Mar 2; 26(3): 153
9. Dissemond J, Lützkendorf S, Dietlein M, Neßeler I, Becker E, Möller U, Thomassin L, Bohbot S, Münter KC. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care. 2020 Jun 2;29(6):350-361. doi: 10.12968/jowc.2020.29.6.350. PMID: 32530781.
10. Augustin M, Keuthage W, Lobmann R, Lützkendorf S, Groth H, Möller U, Thomassin L, Bohbot S, Dissemond J, Blome C. Clinical evaluation of UrgoStart Plus dressings in real-life conditions: results of a prospective multicentre study on 961 patients. J Wound Care. 2021 Dec 2;30(12):966-978. doi: 10.12968/jowc.2021.30.12.966. PMID: 34881999.
11. “In vitro” study. Internal Report. Laboratoires URGO.
